
An adult female Calabar Python 3'1" length, 630g weight, 2004.

A 2-month old neonate Calabar Python showing the typical red and brown
coloration.
The ruler in the picture is 6 1/2 inches in length.
(Updated January, 2010)
The Proposed U.S. Government
Python Ban: A Larger and Potentially Fatal Issue to the Hobby Left Unaddressed:
As I write this, the process to formulate a Bill to ban python importation is still ongoing and some sort of Congressional action may be coming soon. Given the recent apparent inability of Congress to investigate, evaluate facts and/or data, and arrive at reasonable solutions, I would be surprised if a Bill is passed that will come close to doing anything but anger both sides of the debate. The point I would like to make here is that in the midst of all of the "drama with the United States Association of Reptile Keepers actively pushing one viewpoint and the Humane Society of the United States pushing another, everyone is ignoring an issue that in my opinion may eventually do much, much greater damage to the hobby - the importation, maintenance, breeding and sale of highly venomous snakes.
The potential of venomous snakes to become established in many areas of the United States is a very real threat. Places that immediately come to mind include Florida (the center of reptile importation), the Gulf States, Texas, Western United States deserts, and large parts of the West Coast. I believe that this is a much greater threat than constrictors and it is currently being ignored by everyone - the hobby, the Public and Congress. A quick visit to the "kingsnake.com" classifieds indicates that for $2000, you can be the proud owner of a large collection of mambas, Death Adders, Terciopelos, Puff Adders, South American Rattlesnakes, Gaboon Vipers and spitting cobras. Some dealers do state that they will not sell these species to people living in areas that forbid keeping venomous reptiles. I did not, however, see any queries about potential buyers' prior experience with venomous reptiles! No questions as to the housing planned for these animals, the food supply, the size of the collection, or any other factor that would indicate sufficient knowledge to keep these animals safely! I believe that this existing situation is intolerable and may come back to haunt us all at some point in the future. I have spoken to former reptile owners who bought species with little or no negative information from the dealers, or downright mis-information - a sale is a sale, and let the buyer beware. So right now, we are ignoring a situation that could destroy our hobby. Having a 15-foot Burmese eat a small pet dog is bad enough, but having someone quickly die after being bitten by an introduced species of venomous snake could sufficiently panic the Public (and Congress) into enacting extraordinarily intrusive laws that will make maintaining snakes as difficult as maintaining hawks. How many deaths from mambas would have to occur in the Everglades for South Florida tourism to fall off? How many cobra bites in a Western U.S. National Park would have people avoiding trips there? Is there anyone who seriously thinks that such introductions are an impossibility? I would assume that anyone who cares about the future of our hobby will know that changes are needed and the best place for these changes lies in the behavior of dealers - not in Government laws. The exact nature of these changes will be the subject of considerable discussion, but if we do not acknowledge the difference between selling a Milksnake and selling a WC female mamba who may be gravid, there is an excellent chance that extremely unpleasant regulations will be forced upon us. When I first started this home page about 8 years ago, I warned about the continued sales of large constrictors and highly venomous reptiles. There are now three large constrictors established in South Florida and the hobby is scrambling to fight off Congressional Bills. The venomous reptiles are, as far we know, still not an issue. I would suggest that it be preemptively addressed, before public outrage and fear forces our hand.As of
today (1-9-11), there is a chance that we will see a ban on the
importation of
pythons, boas, anacondas etc. The ban may or may not include the
vast majority of boas and pythons that are much smaller. In my
opinion, either outcome
will have exactly the same impact on the ecological situation as not
passing
any bill at all - absolutely no impact. The animals are with us
and the
only question remaining is how much damage they will eventually do to
the environment,
and to the Public's perception of a hobby that involves breeding snakes
and
other reptiles. As far as the environment is concerned, there
will probably be
very weak data and very strong opinions. The "iguana
scenario" where a "pleasant animal" becomes established without
much impact on anything will be put forth by some while others will
advance the "brown tree
snake scenario" where an introduced snake species devastated Guam's
ecology. Most probably, the true impact
will not become evident until much later, and as I said above, the
snakes are already here and passing bills is a waste of time.
Addressing potential future issues while there is still time
would, in contrast, be a very constructive use of our energies.
GENERAL COMMENTS ABOUT THE PAST TWO YEARS:
I
have been keeping snakes for a long time - seriously for about 11 years. During that period of time I have had only
one or two animals die and, I confess, I took this as being the normal state in
a well-maintained collection. Well,
perhaps it is, perhaps I did things wrong during the last two years (things that I am unaware
of), perhaps something major is going on that I am unaware of, and perhaps the
"Law of Averages" finally caught up with me. 2008 began with 16 animals in my
collection and ended with 10 animals (including 2 added during 2007). A total of 8 animals died! There was no real pattern - I lost my
longest-term captives (1.1 WC Calabar Pythons); long-term CB animals (a 1.0
Mandarin Rat Snake and 1.1 Flame Snakes); and 3 recent WC additions, South
American Green Racers (1.1 Phylodryas baroni and 1.0 P. aestiva). The only animal where the cause of death was
obvious was the female Calabar who had an everted oviduct after laying a clutch
of four eggs. 2009 hasn't been much better and
I lost 1.1 Baja Mountain Kingsnakes, 1.1 Calabars, and 0.1 Mandarin
Ratsnakes so the collection is now down to 6 animals. I would add
that I have looked at records of longevity of snakes in captivity and
all of the animals with the exception of a male Calabar were well up
into the range where deaths have been recorded so I am wondering if the
deaths may not have been, at least partly, simply due
to age-related health issues. All in all, though, it has
been an
absolutely awful period for someone who was simply not used to losing
animals...
ABOUT MYSELF:
HERPETOCULTURE
AND CONSERVATION:
The
idea that herpetoculture could and should play a positive role in the conservation
of species has been advanced by a number of people within the hobby. This
is largely based on two factors - the possibility that animals maintained
within the hobby will form a repository of species that could, at some later
time, be introduced into the wild thereby increasing natural populations or
replacing those that have disappeared; and the reduction of collecting
pressures on existing populations through the increased use of captive born
animals. The validity of both of these ideas is, however, very much in
doubt. There are extraordinary difficulties involved in the introduction
of captive animals back into the wild. It is generally agreed that unless
essential precautions are taken over a long period of time, there is a very
real danger of introducing alien microbes into the environment along with the
target species. For example, my collection is housed in one room and
currently contains one wild caught species, the Calabar Python and the
remainder purchased at different times from different breeders. None of
the animals, either wild caught or captive born, have been kept in the type of
cages and/or room that provides fungal, bacterial, and/or viral
isolation. While I have made efforts to use disposable gloves while
cleaning cages and feeding, there have certainly been numerous lapses. It
is therefore probable that all the animals have been exposed to the complete
array of potential disease-causing organisms in the room. The possibility
does exist that the deaths that occurred in my group this past year resulted
from alien microbes attacking species with no natural immunity - or lacking
normal immunity due to long-term adverse health status due to the stress of
captivity. Currently, they are given clean water and parasite free food,
and do not face many of the natural stressors that wild populations have to
deal with. Such stresses in animals reintroduced into the wild may make
them susceptible to opportunistic pathogens they have been formerly exposed to
from other animals that were housed in the same room. More importantly,
releasing these animals back into the wild might allow some pathogen to invade
wild populations of these and other species with possibly devastating
results. A Chytrid fungus and various viruses have been proposed as key
contributors to the global decline of frogs. This follows an alarming
pattern of "emerging infectious diseases" (EIDs) that have affected a
diverse group of animals and plants globally. These pathogens may have
been spread into amphibian populations by tourists, or by the herpetologists
who were studying these animals. As travel becomes more accessible to
larger numbers of people, the global spread of disease causing entities becomes
more of a probability than a possibility - witness the spread of HIV.
Some years ago, I went to the Monte Verde Rain Forest
Preserve in Costa Rica. Until recently, the Preserve was home to the
Golden Toad (Bufo periglenes), which is now extinct due to fungal
infection, and is to be found only on T-shirts and hotel signs. On a
Tuesday morning I made a last check on my snakes, cleaned some cages, and
changed the water. That afternoon I flew to Costa Rica and spent the
night in San Jose. Wednesday morning a car was rented, and I was walking
in the rain forest by 4:00. A single day after I had been dealing with a
fair number of snake species, all of them exotic to Costa Rica, I was walking
through the Park and areas around it. At the sides of the roads in the
region, I would lift the occasional rock or turn a log looking for
snakes. Since I was very conscious of the possibility of my inadvertently
leaving unwelcome microbes in that environment, I had showered before the
flight and taken only clothes that had just been washed. It certainly
didn't reduce the threat to nil, but I felt that it was a prudent
precaution. I wonder, however, how many others (including herpetologists)
have done the same.
Even if we could take the
proper precautions and had animals that were isolated from other species, it
would still be very difficult to successfully introduce a species into the
wild. Long-term captivity and breeding programs result in populations of
animals that may be functionally and behaviorally different from wild
counterparts. Those of us active in the hobby know how easy it is to
breed for various color and pattern traits. At the same time, we are also
selecting for other far less obvious (or invisible) traits. Selective
breeding for prize winning German Shepherd show dogs resulted in the introduction
of hip dysplasia in the breed and there are numerous breeds known to be
associated with adverse health effects. By the very nature of the hobby,
we tend to select animals with large litter or clutch sizes - traits that may
prove to be a disadvantage for snakes in the wild. Similarly, we will
probably select for individuals that eat the food items we have access
to. It would not be surprising if, over time, the average captive Gray
Banded Kingsnake hatchling (Lampropeltis mexicana alterna) takes mouse
pups as first food much more readily than lizards, its usual initial
prey. This behavioral shift would simply reflect the decreased
reproductive potential for those animals that accept only live lizards as their
first food and/or are difficult feeders and therefore either fail to thrive or
do not achieve reproductive maturity as rapidly. Should a change of this
nature take place, it might not be accompanied by any morphological alteration,
and may be welcomed by the hobbyist – but the release of such animals into the
wild might result in total failure if their offspring are not equipped to
accept their normal initial food species.
Recent evidence has been published indicating that captive-reared salmon
raised in aquaculture farms do not survive as well when released into the wild
as do WC fish. The reasons for these
differences are not, as yet, known but it should be apparent that the same
traits that enable animals to survive in an aquaculture environment may be
detrimental when attempting to survive and breed in the natural environments.
THE COLLECTION
TODAY AND PICTURES OF ANIMALS THAT HAVE DIED DURING THE LAST TWO YEARS
(all animals CB):
Calabar Pythons (Calabaria (= Charina) reinhardti)

An adult female Calabar Python 3'1" length, 630g weight, 2004.

A 2-month old neonate Calabar Python showing the typical red and brown
coloration.
The ruler in the picture is 6 1/2 inches in length.
The Calabar Pythons are one of the first species
that
I kept and bred. I have described my experiences
with
the husbandry of these interesting animals in a Section below.
0.1 Mountain Kingsnakes (Lampropeltis zonata
agalma); 11/97


Baja Mountain Kingsnakes, 2006. Male above, female below.
The California Mountain Kingsnake is one of the more
attractive
Kingsnake tricolor species.
1.0 Brazilian Rainbow Boa (Epicrates cenchria
cenchria);
adult, 1/99


The iridescent Brazilian Rainbow Boa is certainly
one
of the most beautiful snakes. It is a fairly popular species
within
the hobby although I remain puzzled as to why it is not even more
commonly
kept. It is an ideal size, relatively easy to maintain, and not
difficult
to handle.
0.0 Flame snake (Oxyrhopus rhombifer inaequifasciatus); neonates, 5/00
June, 2006


Male (died)
Female (died)

Head of male
I was fortunate in being able to purchase two
hatchling
"Flame Snakes" in May, 2000 from Gulf Coast Reptiles in Naples,
FL.
They were part of a clutch of eight eggs laid by gravid female recently
imported from
Paraguay.
Using the keys to species and subspecies found in the "Catalogue of
Neotropical
Squamata; Part I. Snakes" by J.A. Peters and B. Orejas-Miranda (1970),
I determined
that the animals were Oxyrhopus rhombifer inaequifasciatus,a
subspecies
that is found in Paraguay. I have not located any pictures of
this
subspecies on the Internet so I am attaching three - a male, female,
and
head of the male. Note the different coloration between the
female and
the male, the latter having larger red areas dorsally. I have
only seen one
other specimen of this subspecies for sale and that animal was also a
male and was similar in coloration to mine. This species is
rear-fanged but I have
not
located any references to
human
envenomation. They took live mouse pups as first food and I have
continued
feeding them live mice. Based on the lack of coiling when being
handled, at first I assumed that they were not constrictors but I have
observed the male throwing very tight coils around a large
fuzzy prior to eating it so the issue is still undecided. I have
removed mouse pups from the jaws
of
the female and although there was ample evidence of a bite, the mice
have
not exhibited any obvious signs of toxicity. The snakes are are
extremely secretive and remain under the bedding (paper) almost
constantly, emerging only after the lights are out. As they have
matured, they have become less excitable and they have never shown
any
tendency to bite.
I have noted an interesting trait in
both animals. On April 16, 2004, the
female
had what appeared to be a swollen jaw. I checked the male and
discovered
that he too had this "swollen jaw". It appeared as
though
the animals had simply "puffed out" their lower jaw. The jaws
were
firmly closed (no gaping that would indicate any form of infection)
and
the swelling seemed to vary during the course of the day. Since
the
female
had eaten three fuzzies during the night (the male did not eat), I
decided to leave them alone and check them later. As it
turned
out, the "swelling" disappeared and the animals returned to normal
appearance
the next day:

I have noted this behavior additional times and the
swelling has become fairly constant in the female. In December of that year, I
noted that there was also a swelling in the lower part of the neck:

I took the animal to the University of North
Carolina
Veterinary Hospital where it was given a thorough examination by Dr.
Greg Lewbart and Mr. Larry S. Christian. Tissue samples and swabs
were taken and infections, fluid build-up, and solid masses were ruled
out. To date, the
animal's condition has not changed and she continues to eat so I am
simply keeping
track of her appearance and monitoring her weight.
Both animals grew rapidly
- from 6-8g. at
arrival
to approximately 235g. for the female and 300g. for the male at the time of his death. The
husbandry of the animals
are
identical to those I use for corn snakes (70-75°), paper bedding,
hide
box, and water bowl. They are currently housed together in
a 20-gallon
aquarium with plastic mesh tops. As is the case with all of my
aquaria, it has an insertable divider that enables me to track
the feeding of each animals separately. This year, the male
became infected and died with what appeared to be "blister disease" -
there were lesions on its ventrum. The female died some months later with no signs of "blister disease".
Oxyrhopus are being bred by several hobbyists in
Europe and a friend and I purchased two clutches this year. He
was attempting to get them to a sufficient size to be able to eat
new-born
pinkies and at one point he fed them frozen, locally caught, small
skinks. Immediately after that, they became sick and died.
Subsequently, additional neonates were purchased from the same
source and they are currently being converted from lizards (anoles
only!) to mice. I will take possession of animals as soon as they
are taking mice without any problem.
Mandarin Rat Snakes (Elaphe mandarina);
hatchling
- female.

Male Mandarin Rat Snake (deceased) - Picture of current male Mandarin will be coming

Female Mandarin Rat Snake (deceased).
The range of this species of rat snake is very
large and there is considerable variability in the animals coming onto the
market. The male of the pair I own with a friend died this year.
They differed considerably in appearance but we have not been able to learn
anything definite about their parental stocks and therefore do not know whether
this is normal "within-litter" variation, or whether these animals
represent geographical areas.
1.1 African Mole Snakes (Pseudaspis cana);
neonates,
4/04
Male African Mole Snake






1.1 Mussurana (Boiruna maculata); CB 12/06

I purchased a pair of CB Mussuranas this past year. This is a species that I have always wanted to keep. It is a large rear-fanged constrictor with several species and numerous subspecies ranging from Mexico well into South America. These animals have eaten well and have grown considerably in the time that I have had them. They arrived weighing 40+g. and after a year, currently weigh 90+g.
CARE OF CALABAR PYTHONS WITH NOTES ON BREEDING AND EGG INCUBATION:
During the last few years, requests for information about the husbandry of Calabar Pythons (Calabaria reinhardti) have reached me via my home page and subsequent e-mail. Given the apparent interest in Calabars, I have decided to put the following information here in the hope that it may be useful to people who wish to keep these animals.
Taxonomy: In 1993, the herpetologist A. G. Kluge studied the anatomy of this species and concluded that it was closely related to the Rosy Boa (Lichanura trivergitata) and the Rubber Boa (Charina bottae). This finding placed the Calabar Python in the boa subfamily, rather than that containing the pythons. He then combined ("lumped") the three species (Rosy Boa, Rubber Boa, and Calabar Python) into one genus, Charina. Other herpetologists have informed me that while Kluge's conclusion that C. reinhardti is a boa rather than a python is generally accepted, his proposed taxonomic changes are still somewhat controversial although some herpetologists have accepted them and consider the current name for the Calabar Python to be Charina reinhardti. I am not a herpetologist so my views about the purpose of taxonomy are a bit closer to those of the earlier taxonomists - that is, a central purpose of the Linnean classification scheme that was to make order out of the previously haphazard and imprecise way of naming species. The renaming of species that have had the same names for long periods of time may cause confusion, especially among people who aren't aware of the most recent taxonomic changes. An excellent example of the confusion that taxonomic changes can lead to was also generated by Kluge after he concluded that the common Boa (Boa constrictor) and two species of boas found in Madagascar should be placed in the same genus, Boa. The two Madagascar species were Dumeril's Boa (Acrantophis madagascarensis) and the Madagascar Tree Boa (Sanzinia madagascarensis). Here's the problem - since Kluge placed all three species in the genus Boa, he was left with both Madagascar species having the same name (Boa madagascarensis). There are only two ways to address this problem. The first is to change the name of one species entirely as follows:
Old
name
New (scientific)
name
Boa constrictor
Boa constrictor
Boa constrictor
Dumeril's Boa
Acrantophis madagascarensis
Boa madagascarensis
Madagascar Tree Boa Sanzinia
madagascarensis
Boa mandrita
This creates a new species name, Boa mandrita, that bears no
resemblance to its former name and is therefore rather confusing.
The second way to address this problem is to publish
the
paper with your findings but not change the names. The
information
about the evolutionary relationships will then be in the literature
where
interested people will become aware of it, and the silly confusion
caused
by this type of name change will be avoided. Whether
Kluge's findings with Calabaria
made it absolutely necessary to place this species in a new genus
rather than
allowing it to to retain its name (as a boa) is something that will
have
to be decided by herpetologist taxonomists. For now, I'll stick
with
the genus Calabaria. Regardless of the fate of the
scientific
name changes I will, of course, continue to use the common name,
Calabar Python,
rather than "Calabar Boa". A common name is just that - a name
that
is commonly used by many people. The common name certainly may
change
over time, but at this moment it remains the same and there is no
reason to
change it.
Natural history: The Calabar Python is found in West Africa inhabiting areas of moist soil in forest environments. It is a tubular snake with blunt tail and head. It is generally described as a burrowing animal that preys on lizards and small rodents. The method of defense employed by Calabars involves rolling up into a tight ball with its head in the center and the tail exposed. The head is relatively featureless and if you are any distance from it, you quickly discover that it is virtually indistinguishable from the tail. The eyes are small and the same brown color as the scales surrounding it; the mouth is not a visually distinct structure. There is no apparent neck in the adults and the shape of the tail is identical to the head. As an added "attractant" for the tail, almost all of these animals have random patches of white there that may serve to draw attention to that part of the exposed "ball". At one time I believed that these white patches were found in all specimens, but I recently obtained a female that lacks any. The balling defense has been used by all of the snakes I have kept, and there has never been the slightest sign of an intention to bite. Wild caught animals reach a maximum of 3+ feet but it will be interesting to determine the size that captive born animals eventually reach. The adults that I have seen are all patterned with brown and yellow scales. Neonates have a reddish color rather than the yellow for about 1 year, after which, the reddish scales gradually fade to yellow. I have recently been shown pictures of a WC adult that retained the reddish color. These are not as common as the brown animals and whether they represent regional color morphs or simple variants is unknown.
Husbandry: The following information is
derived
from my experience with three long-term wild caught (WC) animals and a
CB neonate added several years ago. The animals were all kept in one 20-gallon
aquarium with a screen top. Using wood and Plexiglas squares as
platforms, I have made the cage tiered. There is a heating pad
under one end of the cage although the animals are never at that spot
except in the days immediately following feeding.
I use paper to line the bottom of the cage. A large, stable,
water dish
is kept in a corner. The animals are kept in a room where the
temperature
remains approximately 75-80° F throughout the year. These conditions appear to be satisfactory and there have
no
problems with disease or refusal to eat I noted, they appear to prefer the cooler
area. As noted below, however, there may be a requirement for a warmer area when animals are gravid. I have
never
cooled the animals down and they have continued to eat during the
entire
year. Many people also recommend keeping the humidity high. I do not do
this
and have not seen any problems with shedding, although the sheds come
off
in several pieces. In all the years that I have kept these
animals,
retained eye caps have occurred only once and were removed with the
subsequent
shed. I feed live or frozen/thawed rodents - no larger than mouse
"fuzzies"
and have never had any problems with the animals eating. I do not
give
them mice old enough to have open eyes. The Calabars are almost
lizard-like
in their feeding - that is, they do not gape their mouths as wide as
many
other
snake species do. They are deliberate feeders and initiate eating
by
"probing" the feeder pups rather than striking. They compress the
food
within their coils and against the sides and floors of the cage.
Their "personalities" and habits are
quite
interesting and lead to some unusual aspects in their husbandry.
They
are prodigious eaters and the 20-25 large fuzzies given per feeding to
the
trio (generally once a week) are invariably gone the next
morning.
Often, this is the result of one snake eating most of the food.
The
next week another snake(s) will do the same and there has been no
trouble
in maintaining the animals in a single cage. The only time they
refuse
food is during the late pre-shed period (they turn a milky blue gray at
this
point) and one or two days before egg laying (although one female ate
some
fuzzies on the morning of the day that she laid three eggs).
Their
weight fluctuates between feedings to a much greater extent than any
other
species I have kept. The intake of water is "record
breaking"
for their size. The water dish has to be constantly refilled and
I
often see them drinking. While they are supposed to primarily be
a
burrowing species, they are seen prowling during the day more often
than
any of the other species I maintain, especially when they are hungry.
Breeding and egg
incubation:
First, a quick note on the sexing of this species. Probing is, of
course, one method and Ross and Marzac list the probe depths of the
sexes as 10-11 scales in the male, and 3 scales in the females.
It would appear that probing is not necessary, however, since only the
males have noticeable spurs lateral to the anal scale.
My original pair of Calabars has
successfully
bred for the last eight years and a newly acquired female bred the
first
year that she was in the group cage. I would like to take credit
for
having come up with clever strategies to allow breeding, but the first
clutch
was a complete surprise. Since then, the animal has produced
clutches
every year. I have observed these animals mating at different
times during the last three years. Mating took place during a
considerable range of months; October in 2004, and September through
November during 2005. Observations were always made when I turned
the lights on some hours after the room had been completely dark:

I happened upon this scene while checking on my animals in the evening
after the lights were out in the snake room. Soon after I took
the picture, they had separated and went back into the hide area below.
The female laid eggs three to five months after
mating.
As the eggs develop internally, the female becomes
swollen
in the posterior third of the body.
When
this becomes noticeable (see immediately below), I transfer it
to a
10 gallon aquarium with a nesting box containing moist potting soil,
and an under cage heater to provide more warmth.

Male (above) and gravid female (1-26-03). Three eggs were laid 17
days
after
this picture was taken.
Another sign of gravidity is that the areas between the scales in the abdomen become readily visible:


Gravid female on the left (four eggs laid 45 days after picture); male
at
right.
Calabar eggs are extremely large in relation to the size of the adult
female:

These are a series of questions that
I
have about the behavior of some of the snakes in the collection.
I
don't have any definitive answers for them, just ideas. If you
know
of references to material that may offer solutions or if you have any
comments,
let me know...
1. Reason for non-breeding in WC animals.
I have successfully bred Calabar pythons - A wild caught (WC) male and
female
purchased in 1993 and 1994 respectively. I housed them together
and
did nothing other than feed them regularly and offer large and
necessary
amounts of drinking water (details of care for this species are found
above).
In 1998, the female became gravid and laid a clutch of normal
eggs.
Every year since then, she has laid eggs. I assumed that
the
four years between her arrival and the first clutch involved
acclimation to
the point where breeding could occur. I also assumed that this
acclimation
was most important for the female for the following two reasons.
The
first is that the production of eggs involves a great expenditure of
energy
(the clutches have been in the range of 30-40% of the female's
pre-laying
weight). It therefore stands to reason that the stress of a novel
environment
would inhibit the female reproductive processes more than the male who
only
has to expend the energy required for a few ejaculates. The
second
reason is rooted in male chauvinism - it's the female who always gets
the
"headache" while the male is raring to go… A friend purchased
a WC female two years ago. On a whim, we placed it in with my
pair.
Our only concern was that the presence of the new female might inhibit
the
breeding of my pair. We were very surprised when both females
laid
clutches of eggs within one day of each other. Is it therefore
possible
that the male is more reluctant to breed in a novel environment than
the
female? The new female had to be receptive and she was in the USA
less
than a year before laying a clutch. Perhaps, at least for some
species,
it is the male that fails to breed rather than a "non receptive"
female.
The question, then, is whether the above assumption is correct, and if
so,
is it true for other species.
On this topic, I would note that
based
on our original assumption, we placed some male Mandarin Rat Snakes
that
were WC in 1999 in with a captive bred female of sufficient size and
age
to breed. The animals showed no signs of breeding and the female
did not become gravid.
2. Question re: yolk formation in non-gravid
females.
Some years ago my viper boa gave birth to a large litter (23).
This
is another species that is rarely bred, and my
breeding
strategy was similar to the Calabars - put a male and female together
and
leave them alone. She never bred again
and subsequently
the male died. The following year after the male died, she
proceeded
to gain weight as she did when she was gravid -
this
species resembles a Gaboon Viper in being short (1 1/2 - 2 feet) and
attaining
a huge girth that virtually prevents it from
coiling.
I was curious about the weight gain since there have
been a fair number of reports of snakes apparently retaining sperm (or
more
likely, fertilized ova) across seasons and then
giving
birth in the absence of subsequent contact with a male. She
attained
a weight of over 1 kg, and then stopped eating
and
began to lose weight. Around this time I noticed small, hard, brown matter in the bedding. I assumed that it
was
dried feces and simply cleaned the cage. One day, I noted that three large egg masses - that is, yolk
material
floating in the water dish, and I realized that
the
brown material was dried yolk. This past year, the identical
thing
happened with a Rainbow Boa kept by a friend and again, masses of yolk
were
deposited in the water dish. This type of event raises a number
of
questions: Why does an ovoviviparous animal
pass quantities
of yolk rather than conserving energy by re-absorption? Does this
ever
occur in the wild or is this phenomenon simply a result of excellent
availability
of food? How common is this phenomenon in
captive
animals?
3. What are "slugs" (infertile eggs).
Snakes sometimes lay slugs. These are soft,
thin
walled "slippery" eggs that succumb to fungus very rapidly. The
only
reference to this phenomenon that I have found is a single note
referring
to them as infertile. I very much doubt that there is any hard
evidence
for this - just an assumption that since the eggs never produce
hatchlings,
they must be infertile. If this assumption is correct, I wonder
what
the mechanism would be that "signals" the female not to bother
depositing
a normal (thick) layer of calcium on the exterior of the egg. It
would
have to be a signal that regulates shell formation and relies on
fertility
as the trigger. The developmental stage of corn snake and ball
python embryos at the time of laying appears to be somewhat similar to
a 48-72 hour
chick embryo. While in the mother, therefore, the embryo is very
small
and immature. How would such an embryo produce enough "signal" to
affect
shell deposition? An equally (or more) attractive hypothesis
would
be that the slugs are fertilized eggs that are laid prematurely,
possibly
as a result of stress. My black milk snake (Lampropeltis
triangulum
gaigiae) female once produced 12 full-size slugs. She mated
with
the male on numerous occasions, so unless one of the pair is infertile,
the
eggs were fertilized. Why then, did she lay these slugs? In
retrospect,
I may not have provided an adequate nesting box. Could this
factor
(and possibly others) have induced stress that was involved in the
production
of slugs? If stress is a factor, there is again the problem of
signals.
In this case, however, one could postulate hormonal imbalance induced
by
stress chemicals.
4. The
question of double clutches.
A number of snake species "double clutch" - that is,
they
lay an initial clutch of eggs, and then after a suitable interval, they
lay
another clutch (no male being introduced between laying). Very
often
(in my collection anyway) the second clutch is not nearly as viable as
the
first - many slugs and few viable eggs. My anerythristic corn
snake
laid 20+ viable eggs and then followed with a second clutch of 10 slugs
and
one thin-shelled egg. What is the mechanism of double
clutching?
I would assume that fertilized (or unfertilized) ova are in some sort
of metabolic
and developmental stasis. Is this phenomena dependent upon
abundant
food between the clutches? Is this something that only happens in
captivity
- in fact, has it been bred into captive animals the same way that we
have
bred chickens that lay an egg(s) every day?
5. Fecundity issues.
I worked with a group of 11 Mexican Milksnakes (Lampropeltis
triangulum annulata). I bred the eight females with the three
males
in an ordered fashion. I waited two or three days before using
the
males after a previous breeding. All females were bred 3 or 4
times.
In all instances, copulation was observed. The end results were:
one
snake laid slugs; one snake laid slugs and viable eggs; one snake laid
eight
viable eggs; and five snakes never showed signs of being gravid.
The
question I have concerns the "whys" of non laying. Are the males
incapable
of breeding as often as we thought they would be? Are the females
differing
in their fertility? The statement "proven breeders" appears on
commercial
lists of snakes for sale. I wonder if this is an indication that
the
successful breeding of these animals may be less common than I thought.
6. Mating behavior.
When some male and female snakes are put together
for
breeding, there is a characteristic twitching on the part of the males
(and
to a lesser extent, the females). This behavior occurs in all of
the
Colubrid species I have observed. What is the reason for this
twitching?
It is the only time that I have observed this behavior. I can
find
nothing in the literature concerning this phenomenon except for simple
description.
This does not occur before or after the breeding season. It may
not occur when two males are put together during breeding season.
In the
case of two male Honduran Milk Snakes (Lampropeltis triangulum
hondurensis),
in cages adjacent to a receptive female, they simply bit each other
when
placed in the same cage.
7. Temporary color change in adult
Calabars.
On November 27, 2002 I weighed and examined my
Calabars
as part of my husbandry routine. At that time, they all appeared
to
have normal coloration. On December 13 I examined the animals and
noted
that the female F1 was in the midst of shedding and seemed to have
developed
a striking color change in the dorsal scales of the first third of her
body
as well as some white scales on the top of the head (see picture on
left
immediately below). I assisted her in shedding and it became
apparent
that the color change was not the result of retained shed - areas that
I
helped remove the shed from had little discoloration, while the major
areas
of discoloration had not retained the shed. Six weeks later (Jan.
16),
the discoloration was still present although greatly reduced (see
picture
on right immediately below). There were no signs of additional
shedding
in the areas that had lightened. The behavior of the animal
appeared
normal and there was no loss of weight. At the time of the second
picture,
the animal was gravid and she laid three eggs on February 12. She
ate
approximately 20 fuzzies on February 13, and in terms of behavior,
appeared
to be perfectly normal. Neither of the other two Calabars (1.1)
in
the same cage showed any sign of color alteration. By March, she
had lost all signs of the color change. The questions are obvious: What
is the nature of a color change with such a rapid onset, and what
induced it.


12-13-02
1-16-03
TENTATIVE ANSWERS:
As a scientist I know that solving biological problems is not a simple process of finding the one absolutely correct solution to the problem at hand. Based on the evidence I have gathered to date, however, the following are tentative answers to two questions originally asked above. Like every answer in science, it remains open to changes resulting from new information and/or observations. But for now, the evidence indicates the conclusions given below:
Viper Boa:
1997
Animals were observed copulating in water once during
April.
Female gave birth Oct. 11. There were 23 live and 2 dead.
Birth
was not observed.
Attempts to feed with pinkies, unscented pinkies,
and
pinkies scented with salamander slime were unsuccessful. A small
number of animals did eat pinkies scented with a leopard frog.
The neonates were maintained in small containers partly filled with wet
sphagnum moss
and a dry area. The animals appeared to have remained exclusively
in the moss. All non-eating neonates were force-fed with
macerated pinkies
through a syringe. The response to this strategy was strange in
that
the neonates lost most of the intubated weight between feeding (at one
week
intervals). In the end, only 2 neonates survived.
Calabar Python

The Calabar in 2000 with her clutch of three eggs.
2003
2/12 - F1 laid 3 eggs in nesting box. Eggs placed
in
incubator at 84° and 90% humidity.
3/31 - F2 laid 4 eggs in nesting box. Eggs placed
in
incubator at 82° and 90% humidity.
4/8 - F1 clutch: (d55) I slit the two flaccid eggs on
top
with an approximately 1cm cut. There was no leakage of yolk.
4/11 - Hatchlings pipped in the egg that had not been
slit
and one of the slit eggs. There was movement in the other egg.
4/12 - Neonate emerged from "non-slit" egg and placed
in
cage (wt. 36.6g); third hatchling pipped.
4/14 - Remaining two neonates emerged from eggs and
placed
in cages. The weights were 39.4 and 37.7g.
5/6 - Discarded egg that was shriveled and had mold growing on
it.
It was the uppermost egg and had collapsed to a greater degree than the
other
eggs.
5/16 - Two eggs slit - movement was detected in both
eggs.
5/18-19 - Neonates emerged.
Neonates did not begin to eat until 3-4 weeks after
hatching.
Rainbow Boa:
2001-20022004-5
Animals observed mating but female has shown no sign of
gravidity. Female eventually "gave birth" to a series of slugs
and
one partially formed embryo.
2000
4/8-4/25 - Animals bred.
6/23 - F4 laid 13 eggs.
7/2 - F1 laid 8 eggs.
Gray Banded Kingsnake:
20002003
4/26-5/21 - successful matings.
7/20 - Laid 4 eggs, 1 good(?), 3 slugs.
8/6 - Laid 4 eggs, 2 good, 2 slugs.
Eggs incubated at 82º, 96%
humidity.
The eggs were not viable.
Mexican Milk Snake:
19992002
4/24; 4/26; 4/27 – Animals mated.
8/2 - Laid 1 egg.
During the second week of August it became apparent
that
she was egg bound. Palpation did not move the eggs and in late
August
she was given to a veterinarian who surgically removed the (6)
remaining
eggs.
10/4 - Normal neonate found hatched.
Black Milk Snake:
2000Sinaloan Milk Snake:
1998
Animals bred on the following days - 3/15; 3/21; 3/28;
3/29;
4/26; 4/30; 5/8-9; 5/12; and 5/15. Activity was
intermittent.
Positive mating occurred on 4/30.
Female ate at various times through 7/3.
7/20-21 - 10 eggs laid in nesting box that contained
sphagnum
moss and peat moss (relatively dry).
7/22 - Eggs weighed 123.5g (female weighed
÷
344.5g). Eggs 36% of pre-laying body weight.
7/27 - Eggs weighed 123.8g. Those on top were
collapsed
to some extent.
7/29 - Eggs weighed 125.0g. Eggs appeared
unchanged.
7/31 - 126.0
8/18 - Eggs appeared unchanged.
9/10 - 146.7
9/17 - Hatching begins (59 days): 4 snakes pipped
9/18 - 3 snakes out of egg
9/19 A.M. - A total of 7 snakes out of egg. 2
pipped.
1 egg (uppermost one) slit with razor. This egg is full, movement
detected.
9/19 P.M. - 2 pipped snakes in morning left eggs.
9/21 - Pinkies given to all 9 snakes.
9/22 - 4 snakes ate
9/23 - Remaining egg slit by snake (away from slit that
I
placed in egg)
1999
Male removed from brumination on 3/19
Animals bred on the following days - 4/18; 4/23; 4/30;
5/4;
5/195/27; 6/6; 6/12; and 6/18. Activity was intermittent.
Positive
mating occurred on 5/27, 6/6, and possibly 6/11.
Female ate at various times through 6/14.
7/1 - 8 eggs laid in nesting box that contained peat
moss
(relatively dry).
8/26-29 - 7 eggs hatched (57-60 days)
Mountain Kingsnake:
20002004
3/19-4/30 - Similar experiences as that of 2003.
2005
The female escaped from her cage during November, 2004 and reappeared
in March, 2005. She appeared to be in good condition although
obviously thirsty. Normally, she begins eating in June, but this
year, her "emergence from brumination" has been delayed and she has
eaten only sporadically through the month. Nevertheless...
6/23 - Female placed with male. Male showed twitching behavior
and attempted to track female.
2004
3/19-4/1 - Animals placed together, male twitched occasionally, female
showed
no interest. Not surprisingly, no eggs were laid.
2005
2/7 - Female placed in cage with male. There did not appear to be
any interest shown by either animal towards the other at any point
after that. A friend and I were, in fact, wondering if we had a
pair, and had decided to have the animals probed. Then...
6/22 - 5 eggs were discovered in cage(!). The eggs were somewhat
collapsed and one has a yellow color and appears to be
non-viable. The eggs were all placed on moist vermiculite in a
covered glass bowl to ensure maximum humidity. A week after
laying, the eggs seem to have filled out somewhat and there is no sign
of fungus.
7/15 - 2 eggs were discarded as they were collapsed and clearly
non-viable. The two eggs were the yellowish one and the one that
was initially the most collapsed. The remaining three eggs were
fully filled out, white, and apparently viable.
8/12 - All three eggs had hatched and the neonates appeared to be
healthy. 2 neonates ate (live pinkies) after first shed.
The third neonate was force-fed Gerber's Beef Baby Food in 1ml amounts
with no problems and there was some weight gain. It refused
food and eventually died.
2006
5/30-6/10 - 5 eggs laid on different days. Only 1 egg appeared to
be normal, all of the others were slugs. Three had been discarded
by 6/15 and one soft shelled egg was kept since no fungus had appeared
as yet. The female did not appear to be done laying (her ventrum
was not "hollow") and I left her in a 10 gal. aquarium with a nesting
box. Soft shelled egg was subsequently discarded.
7/25 - Egg hatched, neonate found in incubator.
8/10 - Neonate refused food, died. My assumption is that it was
not normal at hatching.
2007
No activity noted and no eggs laid.
RECORD KEEPING:
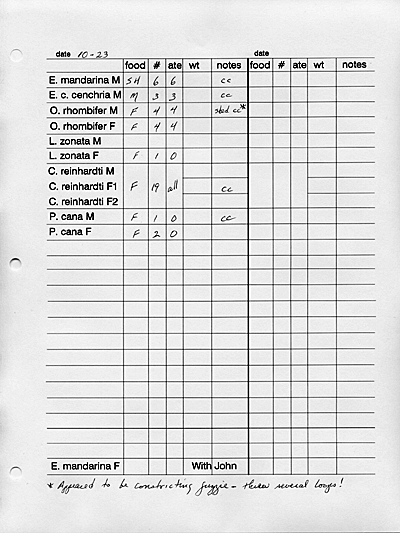
It contains feeding data, shedding information,
weights, and a note
describing an observation made on one of the animals. I store the
data in standard 3-ring notebooks and the format can easily be changed
to reflect changes in the collection.